Innatoss first laboratory to Launch GenScript’s cPass™ test in Europe
Innatoss Laboratories first to Launch Neutralizing Antibody Public Testing Service for SARS-CoV-2 in Europe using GenScript’s cPass™ kit
Innatoss Laboratories has broadened its mission “Catch it early! And significantly reduce healthcare issues associated with infectious diseases” to COVID-19 in the face of the 2020 epidemic.
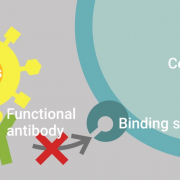
Starting from September 2020 onwards, Innatoss is the first medical lab in Europe that offers testing for neutralizing antibodies against SARS-CoV-2 for the public. Innatoss uses the GenScript’s cPass ™ SARS-CoV-2 Neutralization Antibody Detection Kit. The kit is the first in the world that enables rapid detection of neutralizing antibodies (NAbs), the specific antibodies present in the serum of COVID-19 patients that are responsible for clearing the viral infection.
“Our collaboration with GenScript is a crucial step toward offering high-quality, innovative products to combat the COVID-19 pandemic, which poses a global threat to public health,” said Dr. Anja Garritsen, CEO of Innatoss. “cPass ™ uses a novel test method that is capable of detecting the presence of virus-specific neutralizing antibodies. The test would be useful in determining the longevity of potential immunity both in individuals and the broader population, and facilitating development of vaccines and monoclonal antibody therapy development. It will become the key test to monitor titers once vaccination has been implemented.”
Innatoss recently performed a study in Kessel, a city in the south of the Netherlands that was severely affected during the first wave of COVID-19, to determine the longevity of binding antibodies versus neutralizing antibodies in individuals. The people were initially tested 2-4 months post infection using a rapid test for IgG and IgM levels, and then were retested 2-3 months later. The patients’ serum samples were tested at both time points both for anti-SARS-CoV-2 antibodies by regular ELISA (nucleocapsid protein N and spike protein S1) and for neutralizing antibodies using the GenScript cPass™ kit. In 90 percent of the cases the N and S1 binding antibody levels dropped significantly over time while the level of functional antibodies (neutralizing antibodies) was stable in 70 percent of those tested.
Klick here for more info about cPass functional antibodies in Kessel.
“The cPass ™ test generates reliable results equivalent to the gold standard in virology laboratories, the live SARS-CoV-2 virus neutralization test, without the need for a BioSafety level 3 laboratory,” said Dr. Linfa Wang, the inventor of cPass, and Director of the Programme in Emerging Infectious Diseases at Duke-NUS Medical School, Singapore. “This novel test not only shows people’s infection history, but may provide some indication of protection in the future.”
“This collaboration is a significant step forward, as it offers valuable insights into potential immunity and gives institutions worldwide better access to a reliable COVID-19 testing approach,” said Dr. Patrick Liu, Rotating CEO of GenScript.
Klick here for the article in newswire about the release of cPass.